38 phase diagram with labels
Phase Diagrams - Purdue University You can therefore test whether you have correctly labeled a phase diagram by drawing a line from left to right across the top of the diagram, which corresponds to an increase in the temperature of the system at constant pressure. When a solid is heated at constant pressure, it melts to form a liquid, which eventually boils to form a gas. Iron-Carbon Phase Diagram Explained [with Graphs] - Fractory This phase is stable at room temperature. In the graph, it can be seen as a sliver on the left edge with Y-axis on the left side and A2 on the right. This phase is magnetic below 768°C. It has a maximum carbon content of 0.022 % and it will transform to γ-austenite at 912°C as shown in the graph.
Phase Diagrams - University of Washington sketch a simple eutectic system with limited solid solubility in the end members (components). Label all fields as to phases present. determine the state of the system at any overall composition-temperature point. The state of the system means what phase (s) is/are present, what are their compositions and amounts.
Phase diagram with labels
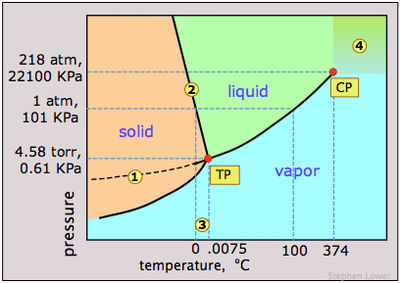
PHASE DIAGRAMS OF PURE SUBSTANCES - chemguide Suppose you have a pure substance at three different sets of conditions of temperature and pressure corresponding to 1, 2 and 3 in the next diagram. Under the set of conditions at 1 in the diagram, the substance would be a solid because it falls into that area of the phase diagram. At 2, it would be a liquid; and at 3, it would be a vapour (a gas). PDF Phase Diagrams States of Matter and Phase Changes Terminology of Phase Diagrams Triple Point The triple point is the location on a phase diagram at which all three lines which divide the three states of matter meet. At this point, all three states of matter may exist at the same time. What is the pressure and temperature for the Phase Diagram of Water - Explanation and Diagrammatic ... - BYJUS A phase diagram is a graphical representation of the various phases of a substance or mixture of substances that coexist in thermodynamic equilibrium, and undergo phase changes under different working conditions, such as temperature, pressure, or volume. The water system is divided into three phases: ICE (S), WATER (L), and WATER VAPOUR (G)
Phase diagram with labels. How to label a blank phase diagram - YouTube Worked example problem solution of how to label single and two phase regions on an unlabeled phase diagram. Also, how to recognize key reactions such as eute... PDF PHASE DIAGRAM - sas.upenn.edu What Label is on the y-axis? 3. List the three phases of matter that are on the diagram. 4. At which point do all three phases on the diagram meet? ... Using the phase diagram of the liquid above, describe any changes in phase present when H 2O is: 12. kept at 0ºC while the pressure is increased from 1 atm to 5atm (vertical line) PDF 1.5 Phase Line and Bifurcation Diagrams - University of Utah For this reason, a sign + or is written on a phase line diagram between each pair of adjacent equilibria. + y 0 y 1 y 2 source sink node Figure 15. A phase line diagram. The labels sink, source, node are borrowed from the theory of uids and they have the following special de nitions:5 Sink y= y 0 The equilibrium y= y 0 attracts nearby solutions at Phase Diagram: Meaning and Types | Material Engineering This phase diagram consists of two points, two lines and three areas. The two points of the two pure metals A & B. The upper line, obtained by connecting the points showing the beginning of solidification is called liquidius line, and the lower line, determined by connecting the points showing the end of solidification is called the solidus line.
What's New in Phase Diagram - FactSage An example of a enthalpy-composition diagram has been added to the Phase Diagram Slide Show . Parameters Window - The Phase Diagram graphical settings that are displayed in the Parameters Window have been expanded and reformatted. Labels & Lines - the line color, line width and line style are new. Phase Diagrams - Chemistry - University of Hawaiʻi We can use the phase diagram to identify the physical state of a sample of water under specified conditions of pressure and temperature. For example, a pressure of 50 kPa and a temperature of −10 °C correspond to the region of the diagram labeled "ice." Under these conditions, water exists only as a solid (ice). Phase Diagram | Explanation, Definition, Summary & Facts A phase diagram is a graphical representation of the substance phases, consists of the curved lines and the space between the two lines represent a specific phase of the matter at given pressure and temperature, whereas any point at the curve lines shows the equilibrium between two phases. Phase diagram explanation Phase diagram - Wikipedia The simplest phase diagrams are pressure-temperature diagrams of a single simple substance, such as water. The axes correspond to the pressure and temperature. The phase diagram shows, in pressure-temperature space, the lines of equilibrium or phase boundaries between the three phases of solid, liquid, and gas .
Phase Diagram - Industrial Metallurgists The phase diagram indicates that an iron-carbon alloy with 0.5% carbon held at 900 °C will consist of austenite, and that the same alloy held at 650 °C will consist of ferrite and cementite. Furthermore, the diagram indicates that as an alloy with 0.78% carbon is slow cooled from 900 °C, it will transform to ferrite and cementite at about ... Phase Diagrams and Phase Equilibria Use this video that describes the phase diagram of water, and phase transformations of water at melting and boiling points, at various atmospheric pressures. Then explore the phase diagram for carbon dioxide. Further, use the tool to understand the triple and critical points in a phase diagram and discuss the phase transitions at these points. Phase Diagram for Water | Chemistry for Non-Majors | | Course Hero Refer again to water's phase diagram ( Figure above ). Notice point E, labeled the critical point . What does that mean? At 373.99°C, particles of water in the gas phase are moving very, very rapidly. At any temperature higher than that, the gas phase cannot be made to liquefy, no matter how much pressure is applied to the gas. 10.4 Phase Diagrams - General Chemistry 1 & 2 We can use the phase diagram to identify the physical state of a sample of water under specified conditions of pressure and temperature. For example, a pressure of 50 kPa and a temperature of −10 °C correspond to the region of the diagram labeled "ice." Under these conditions, water exists only as a solid (ice).
PDF Chapter 9: Phase Diagrams - Florida International University Phase Diagrams • Indicate phases as function of T, Co, and P. • For this course: -binary systems: just 2 components. -independent variables: T and Co(P = 1 atm is almost always used). •Phase Diagram for Cu-Ni system Adapted from Fig. 9.3(a), Callister 7e.
Labeling Phase Change Diagrams | Chemistry | Study.com Steps for Labeling Phase Change Diagrams Step 1: Locate the triple point on the pressure vs. temperature phase diagram. This should look like the intersection of the letter Y . Step 2: Follow the...
PDF 10. Phase diagrams - Campus Tour Fig 10-1 P-T Phase diagram for H 2O To use such a diagram, we locate points in P-T space by specifying values for the two variables. If such a point lies in the area labeled "liquid," that would be the state (and phase) in which H 20 exists. If the point is on the line (e.g., DC), two phases would exist in equilibrium.
10.4 Phase Diagrams - Chemistry We can use the phase diagram to identify the physical state of a sample of water under specified conditions of pressure and temperature. For example, a pressure of 50 kPa and a temperature of −10 °C correspond to the region of the diagram labeled "ice." Under these conditions, water exists only as a solid (ice).
How to Label a Phase Diagram | Chemistry | Study.com Steps for Labeling a Phase Diagram Step 1: Distinguish between the different states of matter at a given temperature and pressure using a phase diagram. Step 2: Use the provided vocabulary terms to...

Electrical Engineering World: The Practical Way of Wiring the Three Phase 60A Distribution Board ...
Phase Diagrams and Phase Equilibria Phase diagrams can be used to understand the stability of different phases of matter (solid, liquid, and gas) under changing temperature and pressure. This lesson plan will help students learn about phase equilibria through the example of the phase diagram of water. Students will learn about the phases of water on Earth, Mars, and Venus and ...
How to label a blank phase diagram - YouTube Phase diagrams are a super helpful resource for materials scientists. Labeling them can be challenging, but, fortunately, there are some simple rules to follow. The top portion will be liquid, the...
PDF Phase Diagrams A typical phase diagram has pressure on the y-axis and temperature on the x-axis. Figure 1: Example of a general phase diagram. The labels on the graph represent the physical state or phaseof the substance at equilibrium under all possible pressure/temperature conditions. The three common physical states are solid, liquid, and gas.


Post a Comment for "38 phase diagram with labels"